Does my project require IRB review?
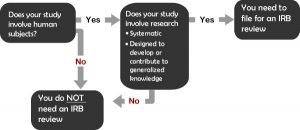
To determine if your project requires review, you will need to first determine that your study meets both the federal definitions of (1) research and (2) human subjects.
- Are you doing research?
- Research is defined as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.”
- Systematic investigations are those that meet all of the following four criteria: (1) There is an attempt to answer a research question or address a hypothesis, (2) data is collected in an organized and consistent fashion, (3) data is analyzed, whether quantitative or qualitative, and (4) conclusions are drawn from the results.
- Generalizable knowledge includes one or more of the following concepts: (1) The knowledge contributes to a theoretical framework of an established body of knowledge, (2) the primary beneficiaries of the research are other researchers, scholars, and practitioners in the field of study, (3) publication, presentation, or other distribution of the results is intended to inform the field of study, (4) the results are expected to be generalized to a larger population beyond the site of data collection, (5) the results are intended to be replicated in other settings, and (6) web-based publication for professional purposes.
- A Note for WOU-Affiliated Faculty, Staff, and Students: Work completed for publication in WOU-based journals and/or repositories (e.g., Digital Commons, Pure Insights, etc.) are considered to contribute to generalizable knowledge and therefore require IRB approval.
- Does the research involve human subjects?
- Human subject is “a living individual about whom an investigator (whether professional or student) conducting research:
- Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospeciments; or
- Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”
- Human subject is “a living individual about whom an investigator (whether professional or student) conducting research:
Use the Human Subjects Research Determination Worksheet to assist you in carefully considering whether your project meets the federal definitions discussed above. If after completing that worksheet you are unsure whether your study requires review or if you need a letter formally documenting that review is not required, submit the form to our office.
If your study does meet the definition of human subject research, you will then need to prepare an initial submission for either an exempt determination or IRB review. Human subject research activities may not begin until an exempt determination or IRB approval is issued by the WOU IRB.
Levels of Review
Studies determined to involve human subject research must be reviewed by the WOU IRB. There are three types of review: Exempt, Expedited and Full Board.
As a part of the application process, individuals designate which type of review they anticipate will be needed, (Exempt, Expedited, or Full Board). ALL Review categories require IRB approval.
Only an IRB member can determine actual review necessary. Researchers are encouraged to contact the IRB prior to submitting an application if they are unsure of their review level.
- Exempt – May take 2-4 weeks to review
- Expedited – May take 4-6 weeks to review
- Full Board – May take 6-8 weeks to review
Please note that full board review requires more extensive processing than exempt or expedited review, including an in-person meeting of the PI with the IRB members so the IRB members can directly discuss the proposal with the PI. This meeting will take place at the next regularly-scheduled meeting of the IRB and will be coordinated with the PI prior to the meeting. The PI should come prepared to answer questions regarding their proposal.
Immediately after meeting with the PI, the IRB members will discuss the proposal and compile a single review form with required modifications, if there are any.
- If the modifications are deemed minimal, after the modifications are completed by the PI and the proposal is re-submitted to the IRB, the IRB members will review the modified proposal. This review process should be expected to take approximately one week.
- If the modifications are deemed substantial, the review process includes another in-person meeting of the PI with the IRB members to discuss the proposal.
Please see the Full Board Review Flowchart for a summary of this process.
Timelines for Review
Timelines for review depend on several factors.
- The level of review required. Exempt review typically takes 2-4 weeks, expedited review 4-6 weeks, and full board review 6-8 weeks.
- The quality and completeness of the submission.
- The number of studies in the queue at the time of submission (studies are processed in the order in which they are received).
- The length of time it takes applicants to respond to pending items and questions for the IRB committee.
- The availability of the IRB members (all members are volunteers).
Please remember no data can be collected until the study receives IRB approval.
Required Training (CITI)
WOU’s human subjects training requirement provides research personnel with the necessary knowledge and understanding of the underlying ethical principles of human subjects research. In order to meet this basic training requirement, WOU uses the online Collaborative Institutional Training Initiative (CITI) training. CITI is a web-based training product that was designed by, updated, and maintained by a number of nationally known IRB professionals.
Who is required to have human subjects training?
- All research personnel working with participants and/or identifiable participant data, including those unaffiliated with WOU, must be listed on the protocol and maintain current human subjects training certification. In addition, faculty members advising students (this includes faculty members not interacting with participants and/or identifiable participant data) on human subjects research must have current CITI training certification.
How do I satisfy the human subjects training requirement?
- WOU students, faculty, and staff listed on a human subjects protocol must take the Group 1: All WOU Researchers training course listed under the CITI course options.
- The minimum passing score is 80% for the entire course. You may improve a score by repeating modules and quizzes. When your Grade Book gives you an option to print a “Completion Report,” it means you passed the course. Upon completion of CITI training, researchers should send proof of completion (e.g., the Completion Report) directly to the WOU IRB.
- CITI certification lasts for three years, and an automated reminder will be sent to certified researchers shortly before current certification is about to expire. To renew certification, researchers must complete the CITI refresher course, Social & Behavioral Research – Basic/Refresher, listed under the CITI course options.
- Research personnel not affiliated with WOU must also have CITI training. Anyone can create an account with CITI and affiliate with WOU. In lieu of the WOU CITI training requirement, the WOU IRB may accept documentation of human subjects training completion in accordance with a researchers’ home institutions’ policy.
Current human subjects training must be in place before engaging in human subjects research activities. The WOU IRB will not approve a project until training requirements are satisfied.
It is the responsibility of the principal investigator to ensure every person on their research team is listed on the protocol and has current human subjects training certification.
The Review Process
Submission
- All required documentation and project materials must be submitted via Submittable, the WOU IRB’s online application and form submission system. The submitting PI will receive a confirmation email and copy of their submission.
- WOU IRB staff reviews documents and materials for completeness and submission requirements.
Pre-Review
- WOU IRB staff reviews protocol materials to prepare for IRB review.
- If staff identify changes necessary before further review can be conducted, an email is sent to the researcher outlining the requested changes.
- If needed, the researcher provides revised materials to the WOU IRB via Submittable.
- WOU IRB staff verifies review readiness, assesses level of review, and sends protocol for review.
Review
- Exempt Review – Protocols are routed to one IRB member for review and verification of exemption. If an exemption is warranted, the PI will be notified and provided with documentation of the exemption.
- Expedited Review – Protocols are routed to two IRB members for review to determine all regulatory approval criteria are satisfied.
- Full Board Review – Protocols are routed to all IRB members and scheduled for review at the next available fully convened IRB committee meeting (see Levels of Review section above for more information).
Post-Review Actions, Communications, & Revisions
- WOU IRB staff communicates review outcome.
- If changes and/or clarification are necessary to secure approval or an exempt determination, WOU IRB staff will contact the PI and provide a comprehensive description of the required clarifications and/or modifications.
- The researcher provides a response to the review and any revised materials (with changes noted/outlined) to the WOU IRB.
- WOU IRB staff verify the changes satisfy the reviewer’s requests. Once satisfied, approval or exempt determination documentation is issued.